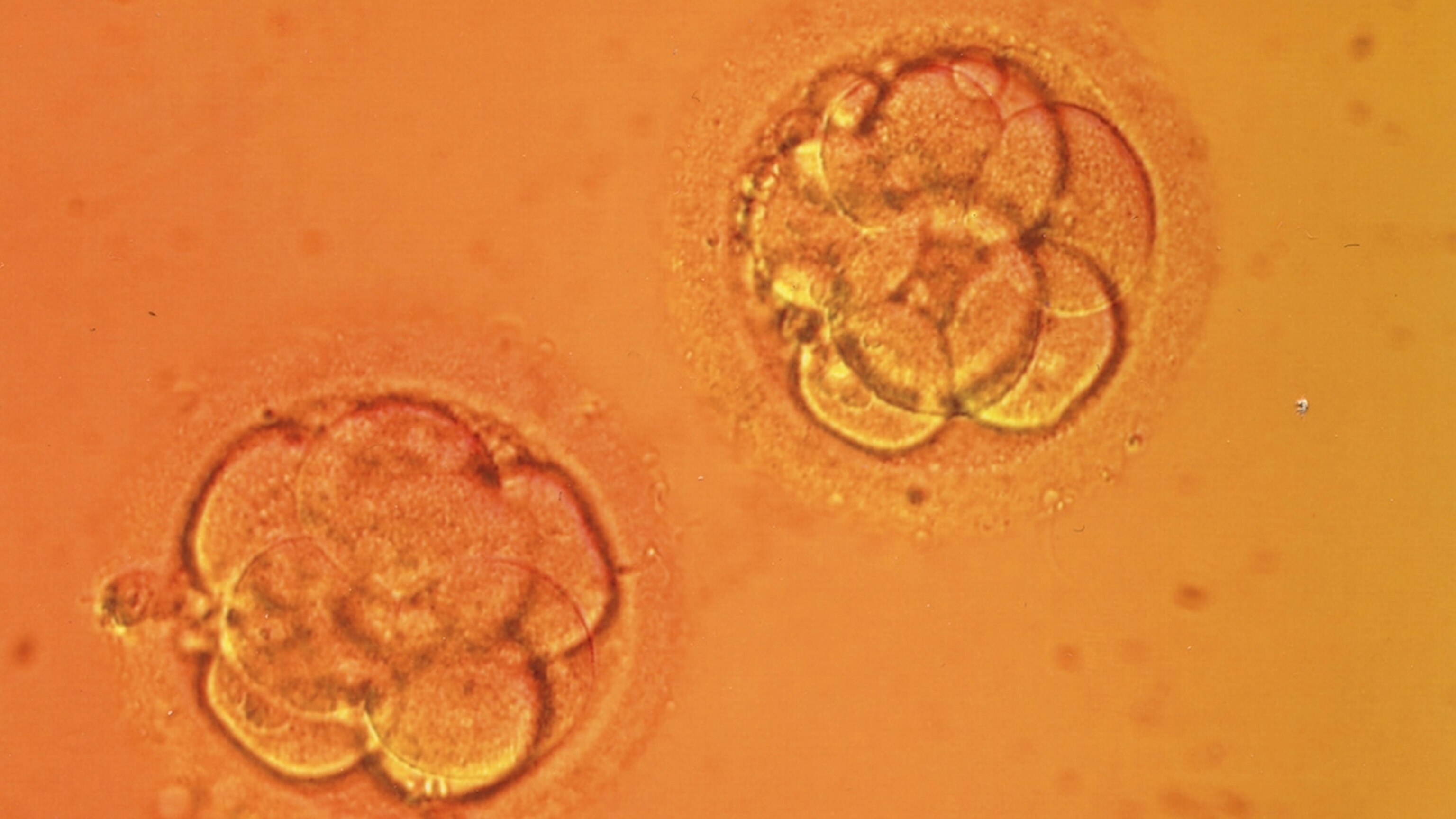
Earlier this week, Chinese researchers reported that they edited the genes of human embryos using a new technique called CRISPR. While these embryos will not be growing up into genetically modified people, I suspect this week will go down as a pivotal moment in the history of medicine. David Cyranoski and Sara Reardon broke the news today at Nature News. Here I’ve put together a quick guide to the history behind this research, what the Chinese scientists did, and what it may signify.
There are thousands of genetic disorders that can occur if a mutation happens to strike an important piece of DNA. Hemophilia, sickle cell anemia, cystic fibrosis– the list goes on and on. As I wrote in the Atlantic in 2013, a particularly cruel genetic disorder, fibrodysplasia ossificans progressiva causes people to grow a second skeleton. It’s caused by a mutation that changes a single “letter” of a single gene, called ACVR1. The protein encoded by the gene doesn’t work properly, triggering a wave of changes in people’s bodies, with the result that when they heal from a bruise, they replace entire chunks of muscle with new bone.
In some cases, people can offset many of the symptoms of genetic disorders with simple changes, like watching what they eat. In other cases, like hemophilia, they have to take regular doses of drugs to remain healthy. In other cases, like fibrodysplasia ossificans progressiva, there’s no effective treatment yet.
For decades, scientists have tried to develop a new way to treat genetic disorders like these: to heal the patient, heal the gene.
This approach came to be known as gene therapy. As I wrote in Wired, gene therapy soared to heady heights of hype in the 1990s. Researchers developed viruses that they could load with working versions of people’s defective genes. They injected the viruses into their subjects, and the viruses delivered the genes to some cells–enough cells, in theory, to start doing the work required to make the people healthy again.
Gene therapy research crashed around 2000 when one volunteer died during a study due to an overwhelming immune response to the viruses he received. Since then, gene therapy researchers have found safer, more efficient viruses, and now gene therapy is starting to emerge in the clinic.
But the revival of gene therapy doesn’t necessarily mean that viruses are the best of all possible tools to fix broken genes. What if, for example, you could just remove the mutant DNA in a gene and replace it with the right sequence?
For a long time, that question was best left to late nights at bars and episodes of Star Trek. Nobody knew how to manipulate DNA with that kind of precision. But in just the past couple years, scientists have created exactly this kind of gene-editing tool, which is known as CRISPR.
As I wrote recently in Quanta, CRISPR didn’t spring fully formed from someone’s mind. It’s actually a collection of molecules that bacteria use to fight viruses. They can create molecules that can latch precisely to certain stretches of DNA and cut them apart. Shortly after scientists figured out how bacteria use CRISPR, they began to wonder if they could use it, too.
It soon became clear they could. They could easily synthesize “probe” molecules that would grab onto a specific stretch of DNA in just about any cell. Enzymes could then chop out that stretch. If the scientists supplied a different version of that stretch of DNA, the cell would incorporate it where the original stretch once was.
Delivering CRISPR into the bodies of people with genetic disorders could conceivably repair their genes. Of course, the success of this kind of treatment would depend on how efficiently the molecules could get inside the cells that needed repairing, and how accurately they cut the DNA. Still, some early experiments on animals suggest that the approach may someday work on people.
But what if you didn’t have to wait until so late in the game to repair a broken gene? If a fertilized egg ended up with a defective gene, you could conceivably use CRISPR to fix the mistake. That single cell could then give rise to an entire healthy human with trillions of cells that all had the correct version of the gene.
Last month, a team of leading scientists–including pioneers in both gene therapy and CRISPR–declared that this would be a bad idea. “At present, the potential safety and efficacy issues arising from the use of this technology must be thoroughly investigated and understood before any attempts at human engineering are sanctioned, if ever, for clinical testing,” they declared in a piece they published in Science.
But meanwhile, a team of researchers led by Junju Huang at Sun Yat-sen University were testing out CRISPR on human embryos. Huang told Nature that both Nature and Science rejected the paper based on ethical objections. So they ended up publishing the results in the journal Protein & Cell (open access, by the way).
The scientists tested out CRISPR as a form of embryonic gene therapy. Imagine an embryo had a mutation in a gene called beta-globin involved in making hemoglobin. It would develop into a person with the blood disorder beta-thalassemia. Would it be possible to cure the embryo by rewriting the gene?
The researchers set out to do the study on human embryos–but they didn’t want to use embryos that might ever actually be able to develop into a fully-formed human being. When fertility doctors fertilize eggs with in vitro fertilization, they sometimes end up with two sperm delivering their DNA into a single egg. These “tripronuclear zygotes” can start dividing as normal embryos do, but their abnormal collection of genes causes them to stop developing when they’re still just tiny clumps of cells. The researchers argue that this failure makes tripronuclear embryos “an ideal model system” for studying CRISPR therapy. (Bioethicists, start your engines!)
All told, the researchers injected 86 embryos, 71 of which survived long enough for them to study. CRISPR only managed to cut DNA in a fraction of the embryos, and in only a fraction of those embryos did cells manage to take up the new version of the target gene (called beta-globin).
Two big problems stick out from the results.
One is the fact that CRISPR sometimes missed its target and inserted DNA in the wrong places. Such a misfire wouldn’t just fail to fix a disease like beta-thalassemia. It could create a disease of its own.
The other big problem is that the embryos that did get edited correctly were actually a mix of edited cells and unedited cells–what’s known as a mosaic. Mosaics can give doctors a lot of headaches, as I’ve written in the New York Times. If fertility doctors used CRISPR to create healthy, hemophilia-free embryos, they’d need to make sure the embryos were repaired by picking off a cell and examining it up close. A cell from a mosaic embryo could give doctors the wrong picture of the embryo as a whole.
The authors conclude their paper by warning that these failures need to be “investigated thoroughly before any clinical application.”
Just because this experiment came out poorly doesn’t mean that future experiments will. There’s nothing in this study that’s a conceptual deal-breaker for CRISPR. It’s worth recalling the early days of cloning research. Cloned embryos often failed to develop, and animals that were born successfully often ended up with serious health problems. Cloning is much better now, and it’s even getting to be a business in the world of livestock and pets. We still don’t clone people, though–not because we can’t, but because we choose not to. We may need to make the same choice about editing embryos before too long.
Postscript 4/23 9:30 am: While I was hammering out this explainer yesterday, I got in touch with Jennifer Doudna, a CRISPR pioneer at Berkeley I wrote about in my Quanta piece, who co-authored the call for putting the brakes on human germline CRISPR research. She got back to me this morning with this to say:
Although it has attracted a lot of attention, the study simply underscores the point that the technology is not ready for clinical application in the human germline. And that application of the technology needs to be on hold pending a broader societal discussion of the scientific and ethical issues surrounding such use.
[Update: fixed the details on the beta-globin gene]
Related Topics
Go Further
Animals
- How can we protect grizzlies from their biggest threat—trains?How can we protect grizzlies from their biggest threat—trains?
- This ‘saber-toothed’ salmon wasn’t quite what we thoughtThis ‘saber-toothed’ salmon wasn’t quite what we thought
- Why this rhino-zebra friendship makes perfect senseWhy this rhino-zebra friendship makes perfect sense
- When did bioluminescence evolve? It’s older than we thought.When did bioluminescence evolve? It’s older than we thought.
- Soy, skim … spider. Are any of these technically milk?Soy, skim … spider. Are any of these technically milk?
Environment
- Are the Great Lakes the key to solving America’s emissions conundrum?Are the Great Lakes the key to solving America’s emissions conundrum?
- The world’s historic sites face climate change. Can Petra lead the way?The world’s historic sites face climate change. Can Petra lead the way?
- This pristine piece of the Amazon shows nature’s resilienceThis pristine piece of the Amazon shows nature’s resilience
- Listen to 30 years of climate change transformed into haunting musicListen to 30 years of climate change transformed into haunting music
History & Culture
- Meet the original members of the tortured poets departmentMeet the original members of the tortured poets department
- Séances at the White House? Why these first ladies turned to the occultSéances at the White House? Why these first ladies turned to the occult
- Gambling is everywhere now. When is that a problem?Gambling is everywhere now. When is that a problem?
- Beauty is pain—at least it was in 17th-century SpainBeauty is pain—at least it was in 17th-century Spain
Science
- Here's how astronomers found one of the rarest phenomenons in spaceHere's how astronomers found one of the rarest phenomenons in space
- Not an extrovert or introvert? There’s a word for that.Not an extrovert or introvert? There’s a word for that.
- NASA has a plan to clean up space junk—but is going green enough?NASA has a plan to clean up space junk—but is going green enough?
- Soy, skim … spider. Are any of these technically milk?Soy, skim … spider. Are any of these technically milk?
Travel
- Could Mexico's Chepe Express be the ultimate slow rail adventure?Could Mexico's Chepe Express be the ultimate slow rail adventure?
- What it's like to hike the Camino del Mayab in MexicoWhat it's like to hike the Camino del Mayab in Mexico