If you could have looked up at the sky 4 billion years ago, you would have seen a sun much dimmer than ours today. And if you looked down at the Earth’s oceans, you would have seen an expanse of bobbing waves.
That’s a problem–a simple one, but a big one. And scientists have been wrestling with it for fifty years.
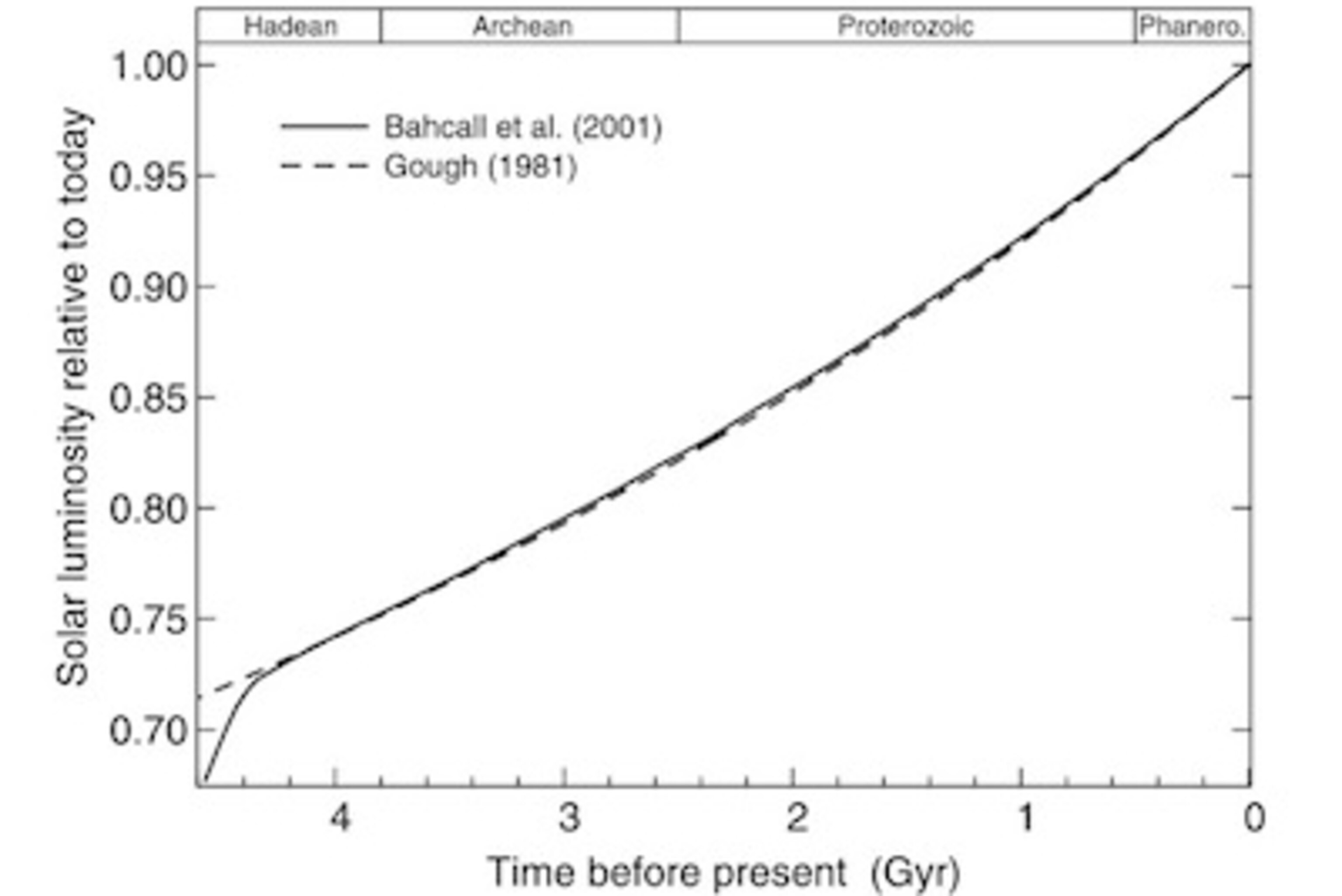
The evidence for these two facts about the early Earth–a dim sun and liquid oceans–were already strong in the 1960s. Astronomers have compared our sun today to other stars of different sizes and ages, and they’ve been able to reconstruct much of its history. The sun started out about 70% as bright as today. It slowly grew brighter; even two billion years ago (2.5 billion years after the Earth formed), the sun was still just 85% as bright as today.
On its own, the faint young sun could not have kept the Earth from freezing over. And yet there are lots of signs in ancient rocks that the Earth was wet. Tiny crystals dating back over 4 billion years have a chemistry that required liquid water. Ancient rocks known as pillow lavas must have formed as molten Earth oozed out into sea water.
As early as the mid-1960s [pdf], scientists realized that these two lines of evidence posed a paradox: what is now known as the Faint Young Sun Paradox. It was a serious problem that required serious thought. It didn’t just mean that the evidence from geology and astronomy wasn’t meshing together. It also added a puzzle to the rise of life on Earth. Life would have had a hard time getting started on a planet of ice.
In 1972, Carl Sagan and his colleague at Cornell George Mullen proposed a solution to the paradox: the greenhouse effect. When radiation from the sun hits the Earth, some of it bounces back into space, but some of it lingers, thanks to heat-trapping gases in the atmosphere. The early Earth would have released gasses from its rocks, creating the first atmosphere. If it had the right chemistry, Sagan and Mullen argued, it might have been able to keep the Earth warm enough to melt ice. They suggested ammonia as a plausible heat-trapper on the young planet.
Unfortunately, ammonia turned out to be a bad solution. Other scientists figured out that ultraviolet rays from the sun would have destroyed any built up ammonia in the atmosphere in less than a decade. That’s not much of a defense against the deep freeze.
But ammonia is not the only greenhouse gas in the game. Today, carbon dioxide and methane are two important molecules keeping our planet warm (and warmer). Scientists have tried for years to narrow down the possible range of the two gases on the early Earth. It’s a very tricky puzzle, because scientists know that there are many factors that can influence their concentrations. And there were factors on the early Earth that we don’t experience today, such as a fairly steady bombardment of comets and giant meteors. Making matters even more complicated, greenhouse gases are not always greenhouse gases. Once the proportion of methane to carbon dioxide gets too high, it produces an organic haze that bounces radiation back into space, cooling the planet.
The consensus today is that methane and carbon dioxide may have warmed the early Earth a fair amount, but not enough to solve the paradox. So scientists are looking at other possible factors. Clouds may have helped. The early Earth rotated quickly through a 14 hour day, which may have changed how the oceans circulated–and thus how they trapped heat. But wide scope still remains for more ideas.
Today in Science, Robin Wordsworth and Raymond Pierrehumbert of the University of Chicago offer two new players to the Faint Young Sun game. They argue that a pair of molecules that have hitherto been neglected–hydrogen (H2) and nitrogen (N2)–could have made up a lot of the difference between the sun’s feeble glow and the Earth’s life-sustaining warmth.
There’s hardly any molecular hydrogen in our atmosphere today, because it easily skips out of the atmosphere into space. But Wordsworth and Pierrehumbert argue that such an escape would have been much harder for hydrogen on the early Earth, partly because it couldn’t get as big of a boost from ultraviolet rays from the sun. They estimate that hydrogen could have made up as much as thirty percent of the atmosphere. They also argue that nitrogen levels were three times higher than today.
On their own, nitrogen and hydrogen don’t do a very good job of trapping the sun’s heat. But when they crash into each other, their structure briefly changes, allowing them to absorb radiation. Wordsworth and Pierrehumbert built a model of a hydrogen and nitrogen-rich early atmosphere and found that as the molecules crashed into each other, they soaked up a lot of heat–enough, they argue, to heat the planet 10 to 15 degrees centigrade. That would go a long way to resolving the Faint Young Sun Paradox.
This new study probably won’t bring the fifty-year debate to a halt. In an accompanying commentary, James Kasting at Penn State argues that nitrogen is too heavy to absorb much radiation, even in the midst of a collision. Instead, Wordsworth and Pierrehumbert are stocking the cabinet that aspiring chefs can raid when they are trying to come up with new recipes for the early Earth.
If hydrogen and nitrogen do turn out to be part of the answer to the Faint Young Sun Paradox, they may have some fascinating implications about life on Earth–and elsewhere. Molecular hydrogen is fine dining for certain types of microbes known as methanogens. As soon as they evolved, they would have been able to feast on a sky full of hydrogen. By devouring the Earth’s protective hydrogen, they might have cooled the planet until it experienced its first glaciers. And beyond Earth, we may need to expand our concept of what kind of planet could support life. If they turn out to have a rich supply of hydrogen and nitrogen, they may offer a toasty incubator for aliens.
Related Topics
Go Further
Animals
- How can we protect grizzlies from their biggest threat—trains?How can we protect grizzlies from their biggest threat—trains?
- This ‘saber-toothed’ salmon wasn’t quite what we thoughtThis ‘saber-toothed’ salmon wasn’t quite what we thought
- Why this rhino-zebra friendship makes perfect senseWhy this rhino-zebra friendship makes perfect sense
- When did bioluminescence evolve? It’s older than we thought.When did bioluminescence evolve? It’s older than we thought.
- Soy, skim … spider. Are any of these technically milk?Soy, skim … spider. Are any of these technically milk?
Environment
- Are the Great Lakes the key to solving America’s emissions conundrum?Are the Great Lakes the key to solving America’s emissions conundrum?
- The world’s historic sites face climate change. Can Petra lead the way?The world’s historic sites face climate change. Can Petra lead the way?
- This pristine piece of the Amazon shows nature’s resilienceThis pristine piece of the Amazon shows nature’s resilience
- Listen to 30 years of climate change transformed into haunting musicListen to 30 years of climate change transformed into haunting music
History & Culture
- Meet the original members of the tortured poets departmentMeet the original members of the tortured poets department
- Séances at the White House? Why these first ladies turned to the occultSéances at the White House? Why these first ladies turned to the occult
- Gambling is everywhere now. When is that a problem?Gambling is everywhere now. When is that a problem?
- Beauty is pain—at least it was in 17th-century SpainBeauty is pain—at least it was in 17th-century Spain
Science
- Here's how astronomers found one of the rarest phenomenons in spaceHere's how astronomers found one of the rarest phenomenons in space
- Not an extrovert or introvert? There’s a word for that.Not an extrovert or introvert? There’s a word for that.
- NASA has a plan to clean up space junk—but is going green enough?NASA has a plan to clean up space junk—but is going green enough?
- Soy, skim … spider. Are any of these technically milk?Soy, skim … spider. Are any of these technically milk?
Travel
- How to see Mexico's Baja California beyond the beachesHow to see Mexico's Baja California beyond the beaches
- Could Mexico's Chepe Express be the ultimate slow rail adventure?Could Mexico's Chepe Express be the ultimate slow rail adventure?
