
Bug Stops Food Halfway Down Its Gut to Make Room for Microbes
Your gut is a long continuous tube. Food goes in one end, gets digested and stripped of nutrients, and is shunted out the other end. That’s the case in ants and elephants, lions and sea lions, hawks and hawk moths. But not in stinkbugs. In the guts of these sap-sucking, shield-shaped insects, food goes in one end, gets digested and stripped of nutrients… and then stops. It never flows into the back half of the gut. That end of the organ has been transformed from a site of digestion, into an apartment complex for microbes.
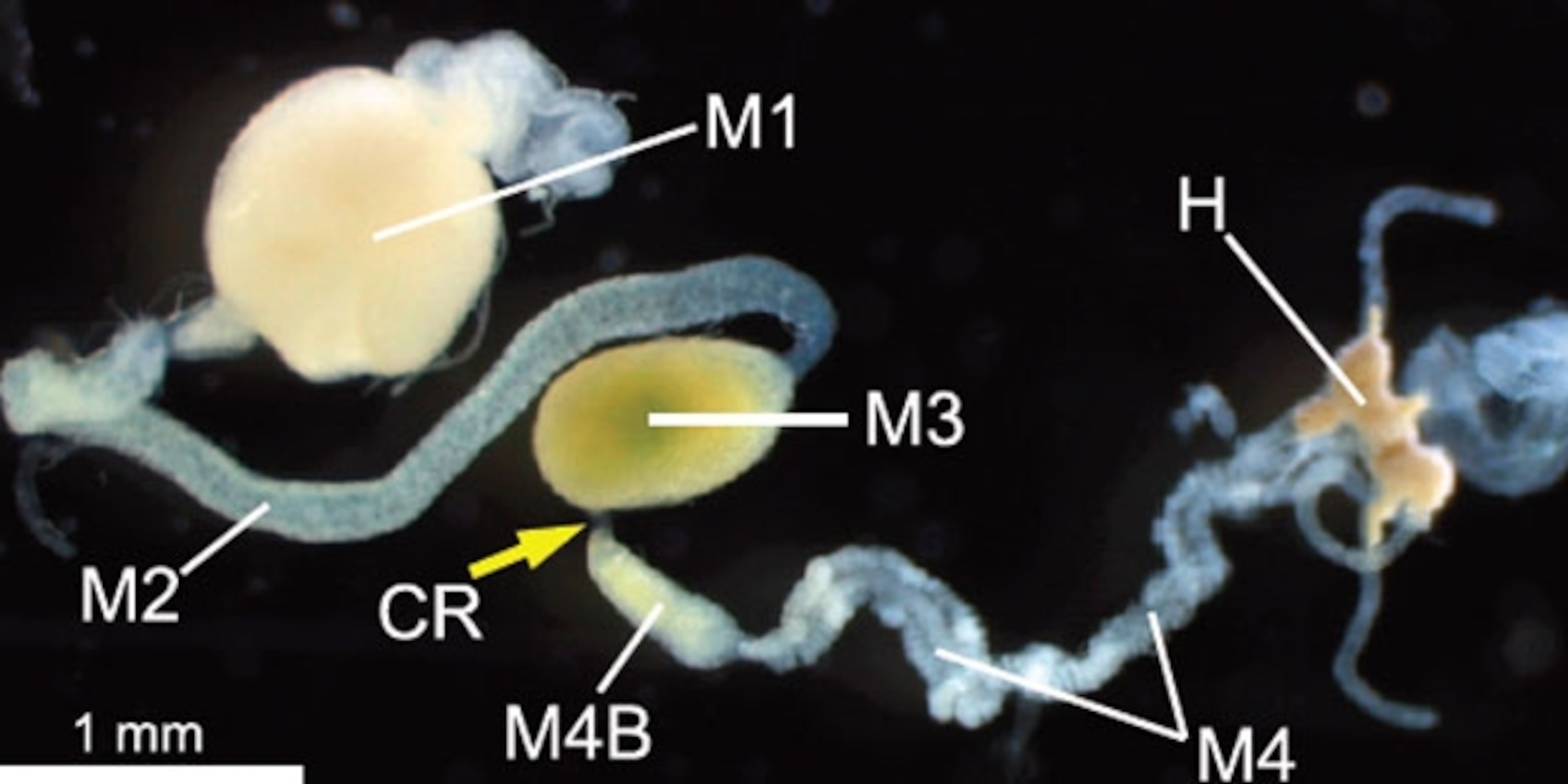
Our guts are full of bacteria and other microbes too, but they live among the currents of food and help us to digest our meals. The stinkbugs have a very different arrangement. Their guts consist of several chambers in a row. The first three (M1, M2, and M3 in the diagram) are for storing and digesting food, and absorbing nutrients. The fourth (M4 and M4B) consists of many branching sacs and crypts, all densely filled with symbiotic bacteria.

Now, Tsubasa Ohbayashi and Yoshitomo Kikuchi from Hokkaido University in Japan have discovered that this separation is enforced by a special organ—an extremely narrow corridor (CR in the diagram) that separates the third and fourth chambers. It’s thin and inconspicuous, which is why no one had noticed it before. But it plays a vital role: it allows certain bacteria to colonise the back half of the gut, while keeping food and other microbes in the front half.
This discovery speaks to two of the most important sides to the wide-ranging partnerships between animals and microbes. The first is conflict. Even beneficial bacteria aren’t an inherent good. They have their own evolutionary interests and can cause severe problems for their hosts if they get into the wrong body parts. So, they need to be contained and controlled. We do so with a wall of mucus that coats our guts, and with immune cells that patrol that wall. Other animals have special compartments, in which they house their bacteria. The back half of a stinkbug’s gut is one example of such specialised living quarters.
That brings us to the second issue: selectivity. The microbes that live in animal bodies aren’t just the same ones the surrounding environment. Only some species have the abilities to thrive in an animal host, and only some are allowed to do so. In stinkbugs, just one bacterium called Burkholderia can colonise the gut. (It’s not entirely clear what Burkholderia does for its host, but we know that it’s important because bugs that don’t encounter it can’t reach their full size and die early.)
The stinkbugs’ newly discovered corridor is responsible for this extreme selectivity. It’s a symbiont sorter. Last year, Kikuchi’s team fed young bean bugs with Burkholderia that had been labelled with a glowing green molecule. They saw that the bacteria formed a queue at the entrance of the narrow corridor and, over several hours, slowly squeezed through. Only Burkholderia does this. Other bacteria can’t make the same journey.
Neither can food or liquid. More recently, the team fed young bedbugs with water that had been stained red with food colouring. The wave of red dye slowly made its way through the gut, and then completely stopped at the narrow corridor. Whatever the organ was, it was impervious to food and liquid, as well as to most microbes.
By studying it under a microscope, the team discovered its secret. For a start, it is impossibly thin: just a few millionths of a metre wide. It is also filled with mucus, which acts as a physical plug. Only Burkholderia can power its way through, partly because it’s a strong swimmer. It propels itself with a powerful whip-like tail, called a flagellum. When Kikuchi’s team engineered mutant Burkholderia that couldn’t assemble a proper flagellum, these strains also couldn’t pass through the corridor.
Then again, other bacteria like E.coli and B.subtilis have their own flagella, and they can’t pass, either. There must be something else that bars their way, and no one knows what that might be. It’s possible that Burkholderia alone can make enzymes that break down the mucus, so that it tunnels as well as swims. Alternatively, Burkholderia might uniquely resist a battery of digestive enzymes and antibacterial chemicals that the bug releases to restrain other microbes.
It’s likely that both the bug and the bacteria have their roles to play in ensuring the fidelity of their partnership. The same is true in other natural alliances. The dinky Hawaiian bobtail squid is colonised by a single species of glowing bacterium called Vibrio fishcerisingle species of glowing bacterium called Vibrio fishceri, which it houses in crypts within its body. Despite the legions of bacteria that swarm in the surrounding seas, only V.fischeri can enter and colonise the squid’s crypts. And as I have written about previously, this selectivity depends on both the squid and the microbe.
The squid and the bug have another thing in common: they both have to get their microbes from the environment with each new generation. So, selectivity is really important to them. They need precise ways of yanking the right partners out of the surrounding milieu. The same is true for most species of stinkbugs, which is why the corridor organ seems to exist throughout the family’s 40,000 or so members.
The only exceptions also prove the rule. Some stinkbugs have evolved very specific ways of handing down the right beneficial microbes to their babies. Some lay capsules full of microbes next to their eggs. Others slather their clutches in a bacteria-rich mucus. Either way, the young bugs are guaranteed to find the appropriate microbes, so the symbiont-sorting corridors in their guts are less useful. So, as is often the way in evolution, they have vanished. When these bugs grow up, their corridor withers into a thread-like strand, and the two halves of the gut essentially become disconnected.
How, then, do these insects excrete waste? Kikuchi’s experiment with the red food colouring revealed the answer. Food gets absorbed in the front half of the gut, channelled to the insect equivalent of kidneys, and then sent back into the very end of the gut to be excreted in faeces. Perhaps that’s as clear a sign as any that these microbes matter. To accommodate them, the bugs have re-routed the entire flow of food in their bodies, bypassing the fourth chamber of their guts, where their bacteria reside.
Reference: Ohbayashia, Takeshita, Kitagawa, Nikoh, Koga, Meng, Tago, Hori, Hayatsu, Asano, Kamagata, Lee, Fukatsu & Kikuchi. 2015. Insect’s intestinal organ for symbiont sorting. PNAS http://dx.doi.org/10.1073/pnas.1511454112
Related Topics
Go Further
Animals
- Octopuses have a lot of secrets. Can you guess 8 of them?
- Animals
- Feature
Octopuses have a lot of secrets. Can you guess 8 of them? - This biologist and her rescue dog help protect bears in the AndesThis biologist and her rescue dog help protect bears in the Andes
- An octopus invited this writer into her tank—and her secret worldAn octopus invited this writer into her tank—and her secret world
- Peace-loving bonobos are more aggressive than we thoughtPeace-loving bonobos are more aggressive than we thought
Environment
- Listen to 30 years of climate change transformed into haunting musicListen to 30 years of climate change transformed into haunting music
- This ancient society tried to stop El Niño—with child sacrificeThis ancient society tried to stop El Niño—with child sacrifice
- U.S. plans to clean its drinking water. What does that mean?U.S. plans to clean its drinking water. What does that mean?
- Food systems: supporting the triangle of food security, Video Story
- Paid Content
Food systems: supporting the triangle of food security - Will we ever solve the mystery of the Mima mounds?Will we ever solve the mystery of the Mima mounds?
History & Culture
- Strange clues in a Maya temple reveal a fiery political dramaStrange clues in a Maya temple reveal a fiery political drama
- How technology is revealing secrets in these ancient scrollsHow technology is revealing secrets in these ancient scrolls
- Pilgrimages aren’t just spiritual anymore. They’re a workout.Pilgrimages aren’t just spiritual anymore. They’re a workout.
- This ancient society tried to stop El Niño—with child sacrificeThis ancient society tried to stop El Niño—with child sacrifice
- This ancient cure was just revived in a lab. Does it work?This ancient cure was just revived in a lab. Does it work?
Science
- The unexpected health benefits of Ozempic and MounjaroThe unexpected health benefits of Ozempic and Mounjaro
- Do you have an inner monologue? Here’s what it reveals about you.Do you have an inner monologue? Here’s what it reveals about you.
- Jupiter’s volcanic moon Io has been erupting for billions of yearsJupiter’s volcanic moon Io has been erupting for billions of years
- This 80-foot-long sea monster was the killer whale of its timeThis 80-foot-long sea monster was the killer whale of its time
Travel
- How to plan an epic summer trip to a national parkHow to plan an epic summer trip to a national park
- This town is the Alps' first European Capital of CultureThis town is the Alps' first European Capital of Culture
- This royal city lies in the shadow of Kuala LumpurThis royal city lies in the shadow of Kuala Lumpur
- This author tells the story of crypto-trading Mongolian nomadsThis author tells the story of crypto-trading Mongolian nomads