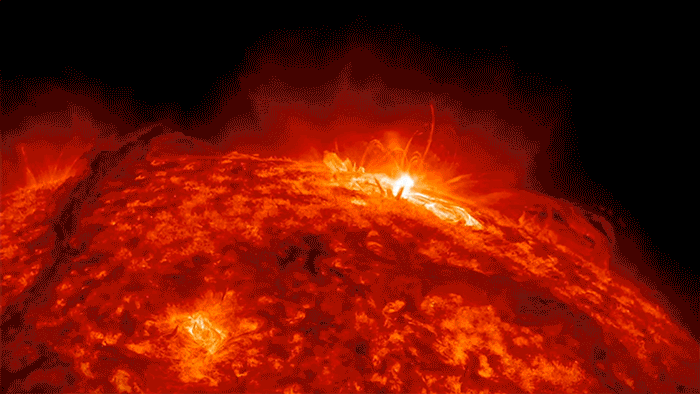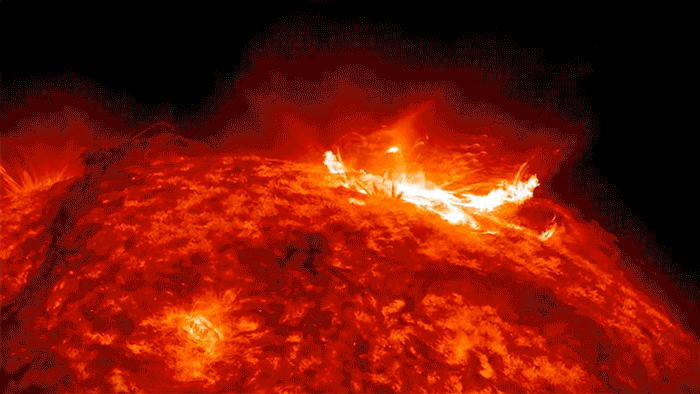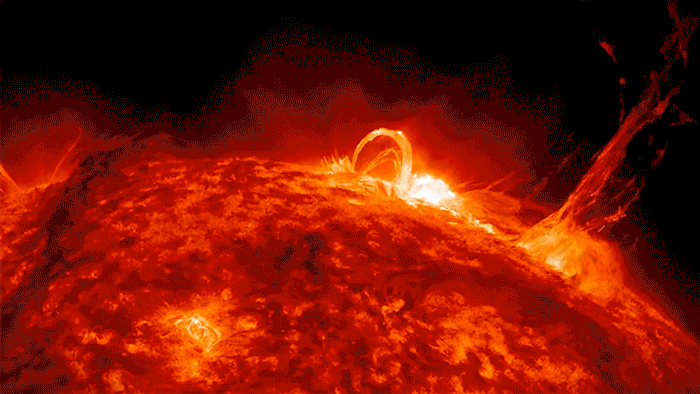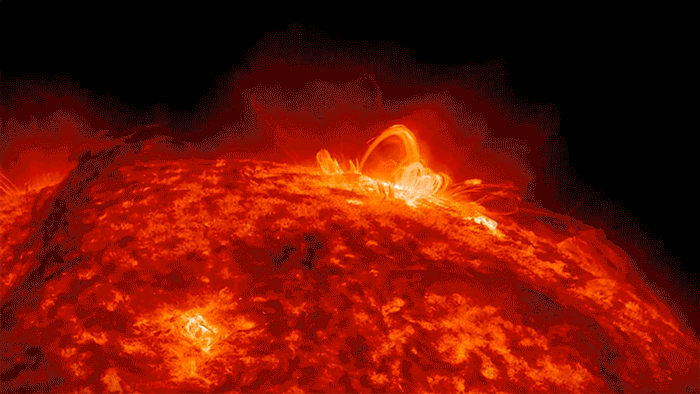
Sunshine’s Crazy Sloppy Path to You
This here?
This is a bit of sunshine. It’s made of pure energy. It has no mass—nothing you can hold, touch, or (accurately) draw. It’s called a photon. Some think of it as a little rat-a-tat of energy packets.
But, being pure energy, it goes so fast (at the speed of light) that its true nature is hard to detect—unless it bumps into something. Here it is colliding with an atom, kicking electrons up into higher, more energetic orbits …

… and then, an instant later, those electrons settle back, energy is released, and —whoosh!—our photons are off again. This is what photons do: They get passed from atom to atom, absorbed and spit out, absorbed and spit out. For those of you who like spice in your lives, be grateful you’re not a photon, especially when you remember that most photons are gathered in dense clumps of burning plasma called stars.
Stars are crammed so tight that atoms get crunched, their electrons stripped away to form vast, free-floating electron clouds. So if you’re a typical photon, you spend most of your time slamming into electron after electron. Fwwaaack! Fwwaack! Fwwack! Your energy is absorbed, then released. You may be able to fly at the speed of light, but because you’re stuck in the middle of the sun, when you finish with one electron, you get to swoosh less than 1/63rd of an inch before you’re absorbed again.
If there were ever a shy photon, one that didn’t like crowds, I imagine that it would yearn to escape the madcap crunch of electrons. I see it deep inside the sun, crawling closer and closer to the surface, electron by electron, until—with any luck—it gets scooped up by one of those giant solar flares and then flung …
… Whoosh! … across the quiet, empty highway of space, careening along at a crazy 670,616,629 miles per hour, free, free at last, like a happy racehorse.
This happens to real photons. Some do get free of their stars, do escape into space as solar radiation. And if they happen to crash into Earth, we call them “sunshine,” and when we go to beaches, lie down, and rub ourselves with lotions, we wait for them to bang into atoms and warm us up.
But consider this: We don’t appreciate how long it takes for sunshine to escape the sun. Every bit of sunshine warming your skin has a long history—wonderfully, fantastically, ridiculously long. Next time you’re at the beach looking up, think about this story.

Imagine A Photon
Let’s start at the very center of the sun, where it’s blazingly hot (10 million kelvins). Here, hydrogen atoms are slamming into each other so hard that their protons fuse and form helium, and with every crash, little bits of pure energy are released. Those are our heros, the photons.
In the crowded middle of the sun, a photon can only move a little way before bumping into another atom. We don’t really know how dense it is in there, but scientists figure our photon hero will zing between a tenth of a millimeter (four thousandths of an inch) to a centimeter (four-tenths of an inch) before its next crash. That’s a crazily small step considering that our photon has to travel 700,000 kilometers to get to the edge of the sun.
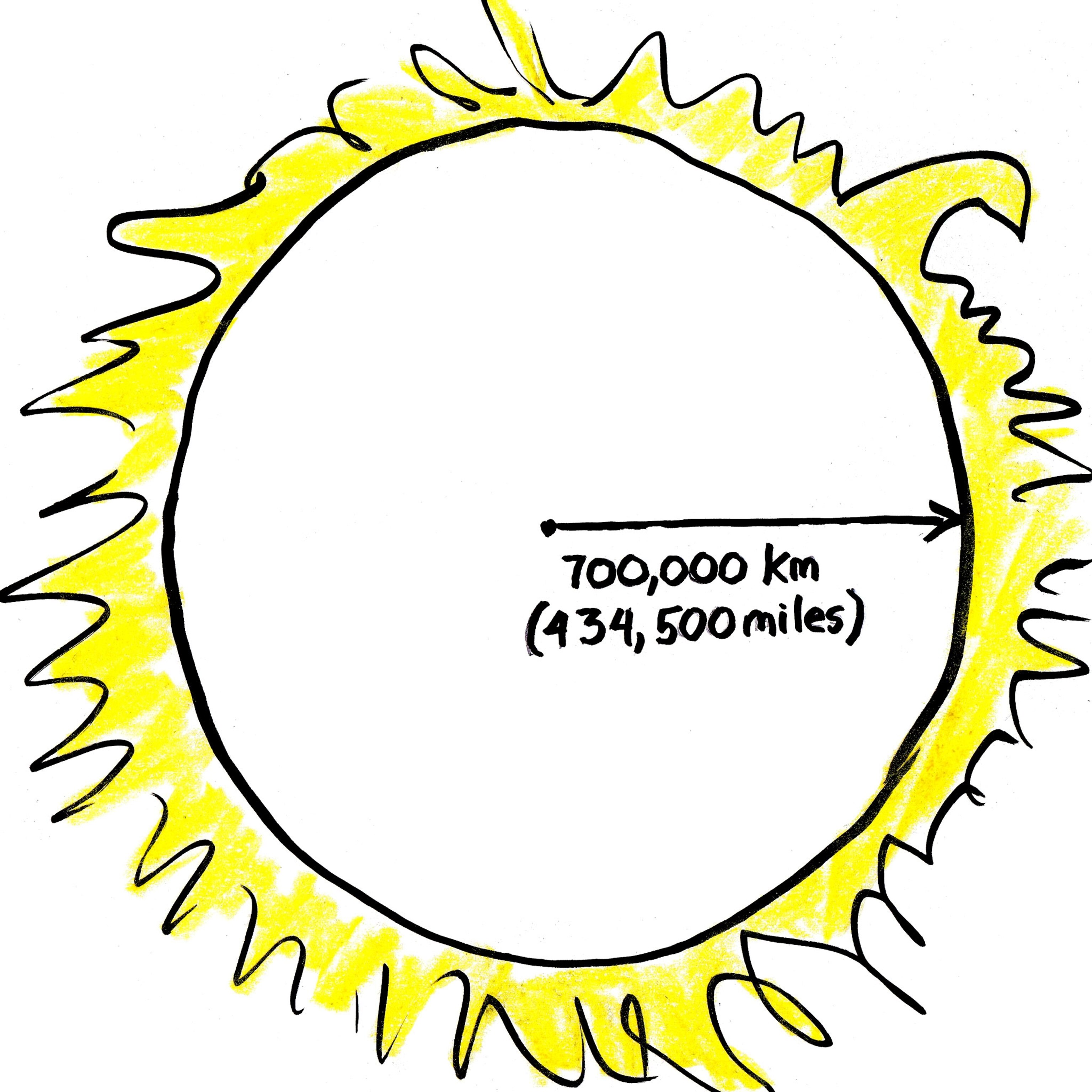
That’s almost twice the distance from the Earth to the moon. So how many steps is it to the surface?
The Nightmare Begins
You don’t want to know. Because here’s the nightmare: When a photon exits an atom, it can go in any direction. It can go up toward the sun’s surface. Or back to the sun’s center. Or sideways. Or any way. Its moves are totally random.
Mathematicians have a name for this: a drunkard’s walk. It describes a guy so drunk that every step he takes is totally arbitrary, and mathematicians have figured out how long it would take this guy, who’s totally blotto, to get from point A (lamppost number one) to point B (lamppost number two).

The answer, writes Richard Gaughan for the blog Synonym, is “that if his starting point and ending point are separated by 10 steps, it will take him, on average, 100 [undirected] steps to get there—that’s 10 squared.” Ten times the steps required.
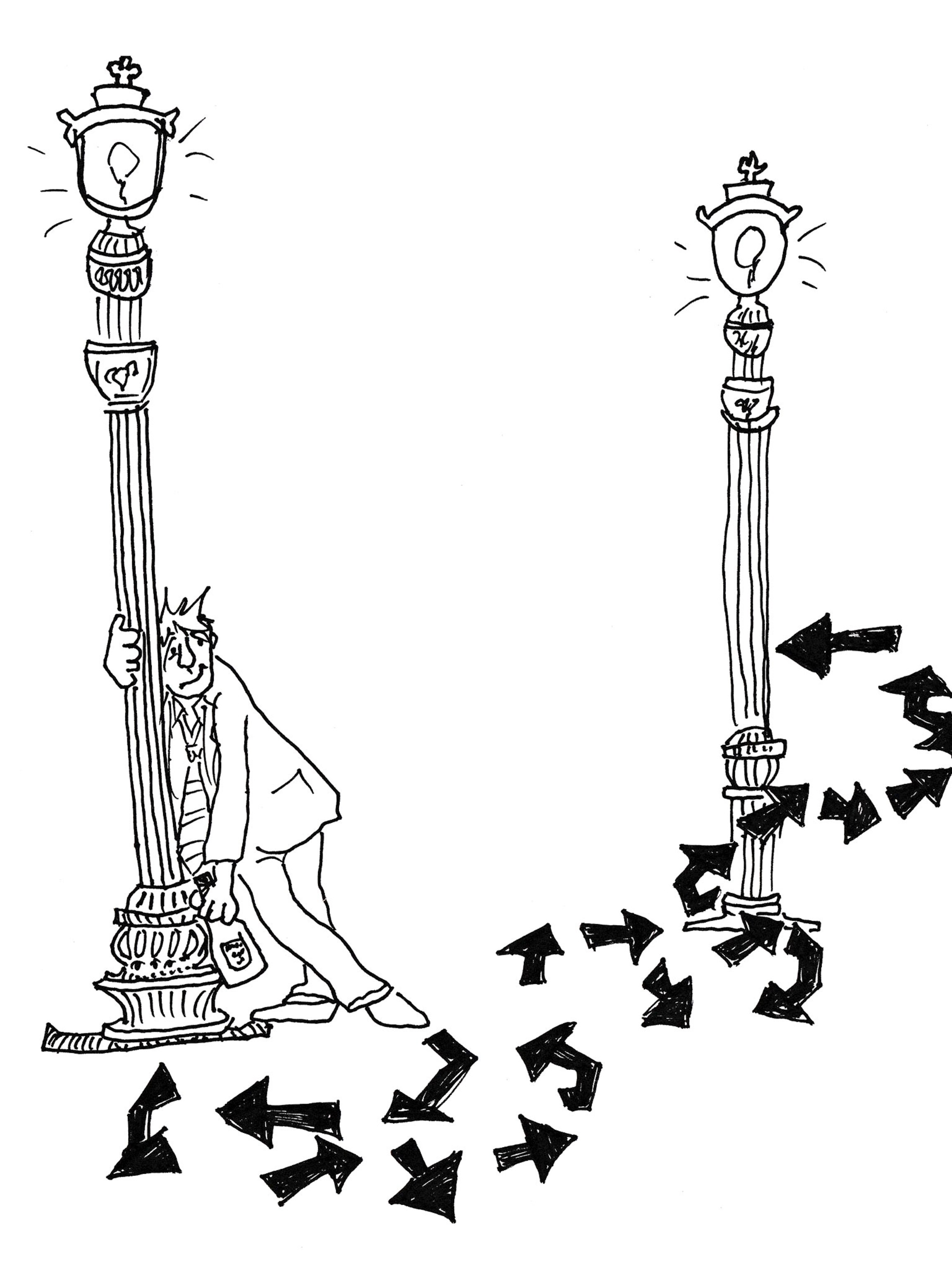
The same goes for our photon. By the time it has zigged then un-zigged, zagged then un-zagged its way to the sun’s edge, it will have had billions, maybe trillions of collisions in every direction.
So to our big question: how long will that take?
49 Trillion Trillion Collisions
Well we can figure this out. If we assume the sun is dense with electrons, and each little “step” is a tenth of a millimeter, to go straight from the center of the sun to the edge will take 7 trillion steps.
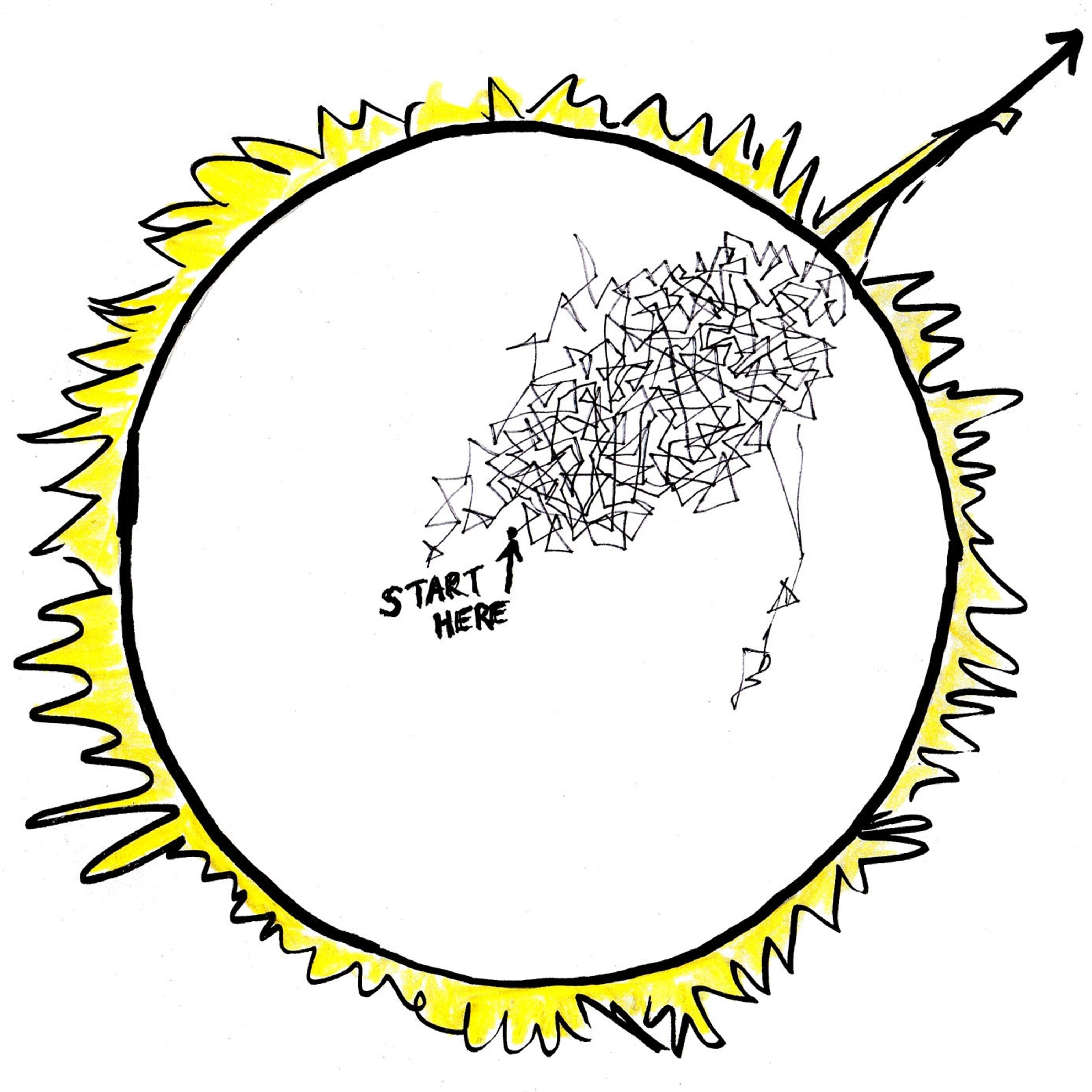
But because our photon is a drunk, its true path will take the square of 7 trillion steps, which works out to 49 trillion trillion collisions before it reaches the surface. Even moving at the speed of light, that will take, more than half a million years.
Half A Million Years!
That’s a long time to wait to become sunshine.
Or …
On the other hand, if we assume a slightly emptier sun, with the traveling distance between electrons a bigger whole centimeter, that works out to fewer steps (only 490 billion trillion) to the sun’s surface—and that in turn works out to journey that lasts roughly 5,000 years.
5,000 years?
But even 5,000 years is a long time. A photon created 5,000 years ago landing on Earth today started its virtual journey around the time the great pyramids were being constructed in Egypt.
If you choose to think about sunshine this way (and I realize photons, being massless, aren’t individuals, and strictly speaking, they can’t be characters in a drama), but if you let the poet in you dance a little, you can go to the beach this weekend, feel the sunshine on your face, and think about how long it took for that to happen.
And whether you’re getting a hit of warmth that’s half a million or 5,000 years old, remember this: Roughly eight minutes ago those photons were still part of the sun, still banging their way through dense throngs of electrons. But once they got flung into space, they raced across the cosmos at the speed of light, wind in their photon hair, and eight or so minutes later, they banged into Earth, and after bouncing through our atmosphere, they settled on you.
Yes, it took a ridiculously long time for photons to get to the edge of the sun, but that last leap to you?
It was short. Crazily, joyously short.
Thanks to Aatish Bhatia for trying to help me with the physics. If I made mistakes, they’re mine, not his, but like a good friend, he tried valiantly to keep me out of trouble. For those of you want a denser look at the math, I recommend “Ask the Space Scientist”, from NASA, where Dr. Sten Odenwald concludes: “…it takes a LONG time for light to leave the sun’s interior.” But here’s a couple of paragraphs…
“The interior of the sun is a seething plasma with a central density of over 100 grams/cc. The atoms, mostly hydrogen, are fully stripped of electrons so that the particle density is 10^26 protons per cubic centimeter. That means that the typical distance between protons or electrons is about (10^26)^1/3 = 2 x 10^-9 centimeters. The actual ‘mean free path’ for radiation is closer to 1 centimeter after electromagnetic effects are included. Light travels this distance in about 3 x 10^-11 seconds. Very approximately, this means that to travel the radius of the Sun, a photon will have to take (696,000 kilometers/1 centimeter)^2 = 5 x 10^21 steps. This will take, 5×10^21 x 3 x10^-11 = 1.5 x 10^11 seconds or since there are 3.1 x 10^7 seconds in a year, you get about 4,000 years.
Some textbooks refer to ‘hundreds of thousands of years’ or even ‘several million years’ depending on what is assumed for the mean free patch. Also, the interior of the sun is not at constant density so that the steps taken in the outer half of the sun are much larger than in the deep interior where the densities are highest. Note that if you estimate a value for the mean free path that is a factor of three smaller than 1 centimeter, the time increases a factor of 10!”
Related Topics
Go Further
Animals
- Octopuses have a lot of secrets. Can you guess 8 of them?
- Animals
- Feature
Octopuses have a lot of secrets. Can you guess 8 of them? - This biologist and her rescue dog help protect bears in the AndesThis biologist and her rescue dog help protect bears in the Andes
- An octopus invited this writer into her tank—and her secret worldAn octopus invited this writer into her tank—and her secret world
- Peace-loving bonobos are more aggressive than we thoughtPeace-loving bonobos are more aggressive than we thought
Environment
- This ancient society tried to stop El Niño—with child sacrificeThis ancient society tried to stop El Niño—with child sacrifice
- U.S. plans to clean its drinking water. What does that mean?U.S. plans to clean its drinking water. What does that mean?
- Food systems: supporting the triangle of food security, Video Story
- Paid Content
Food systems: supporting the triangle of food security - Will we ever solve the mystery of the Mima mounds?Will we ever solve the mystery of the Mima mounds?
- Are synthetic diamonds really better for the planet?Are synthetic diamonds really better for the planet?
- This year's cherry blossom peak bloom was a warning signThis year's cherry blossom peak bloom was a warning sign
History & Culture
- Strange clues in a Maya temple reveal a fiery political dramaStrange clues in a Maya temple reveal a fiery political drama
- How technology is revealing secrets in these ancient scrollsHow technology is revealing secrets in these ancient scrolls
- Pilgrimages aren’t just spiritual anymore. They’re a workout.Pilgrimages aren’t just spiritual anymore. They’re a workout.
- This ancient society tried to stop El Niño—with child sacrificeThis ancient society tried to stop El Niño—with child sacrifice
- This ancient cure was just revived in a lab. Does it work?This ancient cure was just revived in a lab. Does it work?
- See how ancient Indigenous artists left their markSee how ancient Indigenous artists left their mark
Science
- Jupiter’s volcanic moon Io has been erupting for billions of yearsJupiter’s volcanic moon Io has been erupting for billions of years
- This 80-foot-long sea monster was the killer whale of its timeThis 80-foot-long sea monster was the killer whale of its time
- Every 80 years, this star appears in the sky—and it’s almost timeEvery 80 years, this star appears in the sky—and it’s almost time
- How do you create your own ‘Blue Zone’? Here are 6 tipsHow do you create your own ‘Blue Zone’? Here are 6 tips
- Why outdoor adventure is important for women as they ageWhy outdoor adventure is important for women as they age
Travel
- This author tells the story of crypto-trading Mongolian nomadsThis author tells the story of crypto-trading Mongolian nomads
- Slow-roasted meats and fluffy dumplings in the Czech capitalSlow-roasted meats and fluffy dumplings in the Czech capital
- Want to travel like a local? Sleep in a Mongolian yurt or an Amish farmhouseWant to travel like a local? Sleep in a Mongolian yurt or an Amish farmhouse
- Sharing culinary traditions in the orchard-filled highlands of JordanSharing culinary traditions in the orchard-filled highlands of Jordan