[This essay was originally posted on August 13, 2011]
The opening sentence of F. Robin O’Keefe and Luis Chiappe’s new paper in Science this week is a simple statement of fact that threw me for a loop. “Viviparity, or birthing live young,” the paleontologists write, “is common among reptiles, having evolved over 80 times among extant clades.” Think about that for a moment. According to the traditional typology I was taught in my elementary school days, reptiles lay eggs and mammals give birth to live young. Yet monotremes – mammals such as the platypus and the echidna – lay eggs, and clearly reptiles have evolved the ability to produce to live young multiple times. Once again, nature defies our attempts to squeeze everything into neat conceptual boxes.
But modern reptile groups are not the only ones to have shared the evolutionary innovation of live birth. Prehistoric reptiles did, too, as exemplified by an increasing body of fossil evidence documented in a spate of recent papers. Earlier this summer, for example, paleontologists Yuan Wang and Susan Evans reported on a specimen of the approximately 125 million year old lizard Yabeinosaurus which carried at least 15 late-term embryos inside it – a find which pushed the record of live birth in lizards back 30 million years earlier than previously thought. Now the paper by O’Keefe and Chiappe has placed viviparity among a group of marine reptiles whose reproductive lives have remained mysterious since the time of their discovery two centuries ago.
How did plesiosaurs go about making other plesiosaurs? The actual mechanics of plesiosaur intimacy are entirely unknown to us, and, until now, how baby plesiosaurs were born was equally opaque. These prehistoric marine reptiles – four-paddled predators which sculled through the Mesozoic seas – were clearly adapted from terrestrial ancestors into creatures which spent their entire lives at sea. Regarding the birth of baby plesiosaurs, however, it has been unclear whether the marine reptiles gave birth to live young in the ocean, hauled themselves out on the beach to lay eggs, or if baby plesiosaurs were delivered in bundles by pterosaurs. (You know, since there weren’t storks around yet.)
That at least some marine reptiles gave birth in the sea has actually been known for some time. In a 1905 article written for The Century magazine, American Museum of Natural History paleontologist Henry Fairfield Osborn cited two ichthyosaur specimens preserved with embryos as evidence that these marine reptiles were capable of “sea-birth” and did not crawl up on to the beach to lay eggs or went through a “tadpole stage”, as other naturalists had previously proposed. The specimens had come from Holzmaden, Germany – a beautiful graveyard where many exquisitely-preserved ichthyosaurs have been found – and Osborn noted that evidence of fossil embryos within ichthyosaurs had been suspected since 1828. Other cases turned up from time to time, and in 1880 the English anatomist H.G. Seeley published a paper affirming his conclusion that ichthyosaurs were giving birth at sea.

There was some initial uncertainty about this. What if the “embryos” were really the prey of the larger individuals? It’s a reasonable criticism, but the fact that the little ichthyosaurs were consistently the same species as the adults, lacked any evidence of having been fed upon, and were found too far back within the body cavity to have been contained in the stomach or intestines eventually ruled out the cannibalism hypothesis. By the beginning of the 20th century, there could be little doubt that these shark-like marine reptiles were born into the sea. In some fossils the near-term fetus even protruded from the birth canal of its mother – a frozen moment sometimes taken as being the act of birth but that is more likely to be an effect of gases from decomposition pushing the embryo out after death. Many such specimens have been found. While embryos in other ichthyosaur taxa are relatively rare, there are hundreds of specimens of the genus Stenopterygius which contain embryos at varying stages of development.
More recent discoveries have illustrated that ichthyosaurs were not unique in their ability to reproduce entirely in the water. In 2001 paleontologists Michael Caldwell and Michael Lee described a gravid Carsosaurus – a marine lizard known as an aigialosaur which was closely related to the impressive mosasaurs of the Cretaceous. (Imagine a Komodo dragon or monitor lizard suited to life in the water, and you’ll have a good idea of what these creatures were like.) She was carrying four embryos, which – based upon their orientation – were probably delivered tail-first just as in ichthyosaurs, whales, and manatees. Then, just last year, researchers Qiang Ji, Xiao-chun Wu, and Yen-nien Cheng described a Cretaceous reptile called Hyphalosaurus which contained 18 paired embryos. This creature belonged to a diverse group of aquatic, superficially crocodile-like reptiles called choristoderes, most commonly represented by the species Champsosaurus, though it lived in freshwater rather than in the ocean. (In his recent post at Tetrapod Zoology, paleontologist Darren Naish mentions some additional examples, as well.)
Plesiosaurs were briefly on the list, too. In 1887, a few years after he considered the reproductive habits of ichthyosaurs, H.G. Seeley delivered a paper “On the mode of development of the young in Plesiosaurus” at the British Association for the Advancement of Science. Seeley had been given a big nodule of Jurassic mudstone and shale which seemed to contain several plesiosaur specimens, primarily some very small ones which appeared to show “only a slight budding of the fore limbs.” There even seemed to be some vestige of the placenta. These must be embryos, Seeley proposed, meaning that Plesiosaurus was also viviparous. Despite this, however, it seems other paleontologists never took more than a passing interest in the discovery. This may have been for the best. As it turned out, Seeley had been misled by tricky invertebrates.
In 1982 paleontologist Richard Thulborn published a redescription of the specimen Seeley was so struck by. What the 19th century naturalist considered to be embryos were truly “rounded masses of grey-brown mudstone protruding from a core of flaky grey shale.” These bits weren’t skeletons at all – Seeley had thought the specimens were embryos because of their shape alone – but, surprisingly, they were actually still fossils. Though a definite identification was difficult to ascertain, Thulborn proposed that Seeley’s plesiosaur embryos were truly the infilled burrows of prehistoric shrimp.
It turns out that Seeley’s conclusion was right, though, even if his evidence was crap. At least some plesiosaurs delivered their young at sea. The evidence comes from a Late Cretaceous plesiosaur named Polycotylus latippinus which swam the Western Interior Seaway which once cascaded down the middle of North America.
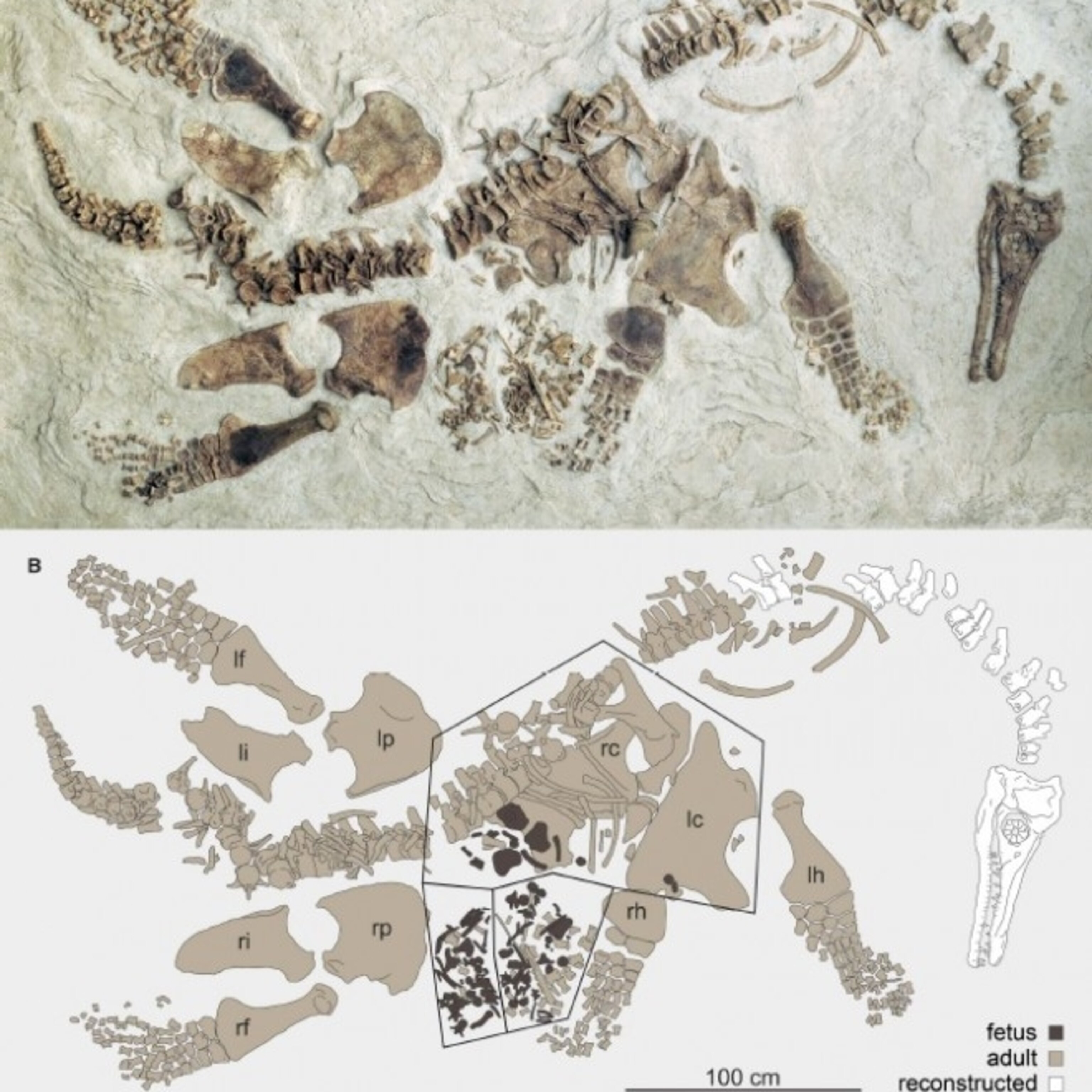
Polycotylus wasn’t your stereotypical plesiosaur. Since the time of their discovery in the early 19th century, plesiosaurs have primarily been represented by long-necked, small-headed forms, but Polycotylus was a relatively short-necked, large-headed variety. The specimen which is the focus of the new paper – LACM 129639 – is a nearly-complete adult splayed out on its bed of Pierre Shale, but near the pelvic region there is a dense accumulation of smaller bones from a young individual of the same species. The smaller animal did not hold together like the embryonic ichthyosaurs of Holzmaden – “The juvenile”, O’Keefe and Chiappe write, “consists of a mass of poorly ossified and largely disarticulated bones spilled from the body cavity of the adult, intermingled with phalanges of the adult right fore-paddle.”
Frustratingly, the specimens were excavated, jacketed, and broken up before anyone drew up a quarry map. Even though the finished fossil looks as if fossil hunters simply brushed off the overlying rock, the whole thing is actually a reconstruction put together on the basis of how the plaster jackets related to each other and observations of the excavators. Still, there are a few clues that indicate that the smaller animal is, in fact, an embryo preserved close the position it occupied inside its mother.
Parts of the embryonic plesiosaur’s hips appear to be preserved in the position they had in life. Two portions of the hip, in particular, were attached to the internal surface of one of the adult’s shoulder bones, indicating that fact that the smaller animal was inside the adult at the time of burial and had not somehow become accidentally mixed up with the larger animal.
So far, so good. But might the small animal have been a meal for the adult? Paleontologist Ken Carpenter raised that possibility in a brief comment printed at ScienceNOW, and almost every case of fossil embryos found inside their possible mothers has raised the possibility of cannibalism. (For another recent example, see the discussion about the small bones found inside the early whale Maiacetus published at PLoS One.) As O’Keefe and Chiappe point out, though, the development of the bones is consistent with the hypothesis that the animal was a fetus and there is no sign that the bones were bitten or etched by digestive acids. The newly-described find is consistent with what has been reported for other pregnant marine reptiles, and there does not appear to be any direct evidence that the small Polycotylus was the last meal of the adult.
There’s more to the paper than a confirmation that plesiosaurs likely gave birth at sea, though. The Polycotylus fetus was large and all by itself – the mother was not carrying a litter of puny plesiosaurs. In terms of natural selection, this is putting all your eggs in one basket. A single, large offspring is a big energetic investment and the sole genetic representative of its parents, and therefore it would be expected that parents which provided some amount of extra care would better ensure the survival of their offspring and the continued replication of their genes. The authors are tentative about the idea, but single, big offspring are often associated with additional maternal care after birth – much like whales provide to their young – and there is the possibility that plesiosaurs did the same.
The ability to give birth to live young in the water is something that has evolved over and over again among disparate groups of marine organisms. Even if we restrict ourselves to the marine reptiles listed in this past – ichthyosaurs, aigialosaurs, and plesiosaurs are all representatives of distantly-related lineages which became adapted to life in the sea independently. Mammals – whales and manatees – also became adapted in similar ways due to their shared way of life. Why, then, are there no penguins or crocodiles that can give birth to live young in the water?
Both penguins and crocodiles are members of a group called the Archosauria – “the ruling reptiles.” (Dinosaurs are included in this group; penguins are the modern day descendants of feather-covered theropod dinosaurs, after all.) There are no live-bearing archosaurs today, but perhaps there were in the past. Fossil evidence has discredited the idea that dinosaurs may have delivered live young, but there was once a long-lived and widespread group of entirely aquatic, sea-dwelling crocodiles. Based on their anatomy and mode of life alone, it is a reasonable hypothesis that these archosaurs may have become adapted to giving birth at sea. Further consultation of the fossil record will be needed to test this idea.
Extinct marine crocodiles aside, though, the ability to bear live young has evolved so many times that it is peculiar that there are no living archosaurs can do the same. Researchers Daniel Blackburn and Howard Evans considered why this might be so in a 1986 paper called “Why are there no Viviparous Birds?” They discounted the notion that there might be some unknown, live-bearing species of birds hiding somewhere in New Guinea, Madagascar, or the Amazon Basin. There must have been some kind of evolutionary constraint which prevented the evolution of the trait.
A variety of different constraints had been considered before. Maybe carrying internal offspring would have weighed down mother birds too much and made them more vulnerable to predators, or perhaps the type of hard-shelled egg birds lay is not able to be adapted into a live-birth reproductive system, among other possibilities. Yet Blackburn and Evans rejected the previously-aired hypothesis. (In the flight hypothesis, for example, the scientists pointed out that female birds do internally carry the eggs before they are laid, and that pregnant bats fly around just fine while carrying the extra mass of their offspring.) Instead, they proposed that there was never any kind of evolutionary pressure that would have favored the internal retention of the egg and internal hatching (one of the modes of live birth). If it ain’t broke, don’t fix it.
Asking about the evolutionary road not taken is tricky business. We can approach the puzzle of why a certain trait or adaptation appeared through the records organisms contain in their anatomy and – among living and recent species – their genes, but how can we really know why this or that feature did not appear? Was there some kind of anatomical or physiological constraint? Did the proper selective pressure just never come into play? Was there more than one cause? These are the kinds of questions that can keep an evolutionary theorist up at night, dreaming of penguins delivering their chicks in the tranquil blue of the sea.
Top Image: The reconstructed skeletons of a mother Polycotylus latippinus and her unborn infant. From O’Keefe and Chiappe, 2011.
References:
Blackburn, D., & Evans, H. (1986). Why are there no Viviparous Birds? The American Naturalist, 128 (2) DOI: 10.1086/284552
Caldwell, M., & Lee, M. (2001). Live birth in Cretaceous marine lizards (mosasauroids) Proceedings of the Royal Society B: Biological Sciences, 268 (1484), 2397-2401 DOI: 10.1098/rspb.2001.1796
Ji, Q., Wu, X., & Cheng, Y. (2010). Cretaceous choristoderan reptiles gave birth to live young Naturwissenschaften, 97 (4), 423-428 DOI: 10.1007/s00114-010-0654-2
Maxwell, E., & Caldwell, M. (2003). First record of live birth in Cretaceous ichthyosaurs: closing an 80 million year gap Proceedings of the Royal Society B: Biological Sciences, 270 (Suppl_1) DOI: 10.1098/rsbl.2003.0029
O’Keefe, F., & Chiappe, L. (2011). Viviparity and K-Selected Life History in a Mesozoic Marine Plesiosaur (Reptilia, Sauropterygia) Science, 333 (6044), 870-873 DOI: 10.1126/science.1205689
Organ, C.; Janes, D.; Meade, A.; Pagel, M. (2009). Genotypic sex determination enabled adaptive radiations of extinct marine reptiles Nature, 461 (7262), 389-392 DOI: 10.1038/nature08350
Thulborn, R. 1982. Liassic plesiosaur embryos reinterpreted as shrimp burrows. Palaeontology 25 ( 2), 351-259
Wang, Y., & Evans, S. (2011). A gravid lizard from the Cretaceous of China and the early history of squamate viviparity Naturwissenschaften DOI: 10.1007/s00114-011-0820-1
Go Further
Animals
- This ‘saber-toothed’ salmon wasn’t quite what we thoughtThis ‘saber-toothed’ salmon wasn’t quite what we thought
- Why this rhino-zebra friendship makes perfect senseWhy this rhino-zebra friendship makes perfect sense
- When did bioluminescence evolve? It’s older than we thought.When did bioluminescence evolve? It’s older than we thought.
- Soy, skim … spider. Are any of these technically milk?Soy, skim … spider. Are any of these technically milk?
- This pristine piece of the Amazon shows nature’s resilienceThis pristine piece of the Amazon shows nature’s resilience
Environment
- This pristine piece of the Amazon shows nature’s resilienceThis pristine piece of the Amazon shows nature’s resilience
- Listen to 30 years of climate change transformed into haunting musicListen to 30 years of climate change transformed into haunting music
- This ancient society tried to stop El Niño—with child sacrificeThis ancient society tried to stop El Niño—with child sacrifice
- U.S. plans to clean its drinking water. What does that mean?U.S. plans to clean its drinking water. What does that mean?
History & Culture
- Séances at the White House? Why these first ladies turned to the occultSéances at the White House? Why these first ladies turned to the occult
- Gambling is everywhere now. When is that a problem?Gambling is everywhere now. When is that a problem?
- Beauty is pain—at least it was in 17th-century SpainBeauty is pain—at least it was in 17th-century Spain
- The real spies who inspired ‘The Ministry of Ungentlemanly Warfare’The real spies who inspired ‘The Ministry of Ungentlemanly Warfare’
- Heard of Zoroastrianism? The religion still has fervent followersHeard of Zoroastrianism? The religion still has fervent followers
Science
- Here's how astronomers found one of the rarest phenomenons in spaceHere's how astronomers found one of the rarest phenomenons in space
- Not an extrovert or introvert? There’s a word for that.Not an extrovert or introvert? There’s a word for that.
- NASA has a plan to clean up space junk—but is going green enough?NASA has a plan to clean up space junk—but is going green enough?
- Soy, skim … spider. Are any of these technically milk?Soy, skim … spider. Are any of these technically milk?
- Can aspirin help protect against colorectal cancers?Can aspirin help protect against colorectal cancers?
Travel
- What it's like to hike the Camino del Mayab in MexicoWhat it's like to hike the Camino del Mayab in Mexico
- Follow in the footsteps of Robin Hood in Sherwood ForestFollow in the footsteps of Robin Hood in Sherwood Forest
- This chef is taking Indian cuisine in a bold new directionThis chef is taking Indian cuisine in a bold new direction
- On the path of Latin America's greatest wildlife migrationOn the path of Latin America's greatest wildlife migration
- Everything you need to know about Everglades National ParkEverything you need to know about Everglades National Park
